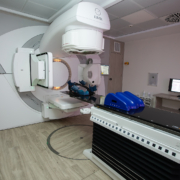
Radiotherapy is one of the most powerful and advanced medical treatments in the world, and specialist oncologists will create individual and highly precise treatment plans to maximise its effectiveness using minimal doses.
When you travel to a specialist international radiotherapy centre, the goal is to treat complex conditions using highly accurate computerised planning, highly accurate external beam radiation and a holistic process for maximising preparation and rehabilitation.
In general, radiotherapy is undertaken as a single course of treatment either delivered over multiple sessions or cycles. In some cases, such as stereotactic radiosurgery, the dose of radiation is so precise and so powerful that the entire treatment can be completed in a single day.
However, one question that we are often asked, particularly in cases of recurrence, is whether radiotherapy can be used in the same area multiple times. Can you have radiotherapy target a tumour in the same part of the body a second or even a third time?
The short answer to this is yes, according to some of the limited number of studies in the area, but exactly when or where it is appropriate to do so will depend on a huge number of factors and will almost invariably need to be undertaken at a specialist centre.
To understand why, it is important to know what repeat radiotherapy is, what it is not, and why decisions to do another course of radiotherapy can be particularly complex.
What Is Repeat Radiotherapy?
Repeat radiotherapy, sometimes known in medical literature as reirradiation, is when a second course of radiotherapy treatment is given to the same area, where there is an overlap between treatments or where radiotherapy will affect the same organ.
It must be stated that repeat radiotherapy is not multiple doses of radiation undertaken as part of a single treatment. Most radiotherapy treatments are administered using multiple doses across several days, weeks or even months, but this does not count as repeat radiotherapy.
Instead, repeat radiotherapy is when an entirely new course of radiation targets the same area, and it is often a decision undertaken with a lot of care, consideration and often a specialist multidisciplinary team separate from the existing cancer care team.
Where Can Repeat Radiotherapy Be Used?
Because precise dose control and placement of radiation are such a vital aspect of repeat radiation, it is most commonly used in parts of the body in which the positions and placement of organs and tissue do not change as much.
These include treatment for:
- Brain tumours and related conditions
- Central nervous system tumours.
- Head and Neck tumours.
- Breast cancer.
- Solid tumours that are easier to plan treatments for.
Whilst not a firm rule, repeat radiation is almost never considered until at least six months have passed since the last dose.
Why Has Repeat Radiotherapy Become More Common?
Historically, repeated radiotherapy was considered to be almost, if not outright, impossible due to the accuracy required for doses and placement of radiation beams.
As both imaging technology principles, such as in-vivo monitoring and advances in linear accelerators, allow for more accurate and safer doses of radiation than ever before, repeat radiotherapy has become a possibility for an increasing number of conditions.
It is still relatively rare and needs to be considered on a case-by-case basis, but it allows for many more options for treatment without the need for conventional surgery.
What Do Oncologists Consider Before Repeat Radiotherapy?
- Whether the person is the right fit to benefit from repeat radiation.
- Whether the tumour or condition would be treated through repeat radiation.
- Previous radiotherapy doses.
- The original distribution and potential dosage constraints for repeat radiotherapy.
- The particular radiotherapy technique used.
Why Is Repeat Radiotherapy Undertaken?
Radiotherapy is delivered in carefully controlled doses to ensure that they do not overwhelm the ability of healthy tissue to regenerate and recover. This is why a considerable amount of time is left between the last dose of your original treatment and any potential repeat doses.
As with any other type of major oncological treatment, it is only even considered if it will provide greater benefits to you than the effects of treatment, something that has only become consistently possible in the last decade.
A second or third round of radiotherapy needs to be treated differently from the first, and will only be attempted by specialists.
What Are The Alternatives To Repeat Radiotherapy?
- Chemoradiation, a combination of a lower dose of radiotherapy and chemotherapy to enhance the effects of the latter.
- Immunotherapy or targeted drugs.
- Novocure, also known asTTFields treatment.